AI for breast cancer diagnostics
Elevate your pathology lab with Aiforia® Breast Cancer Suite – enhancing precision, speed, and diagnostic accuracy with cutting-edge AI technology.
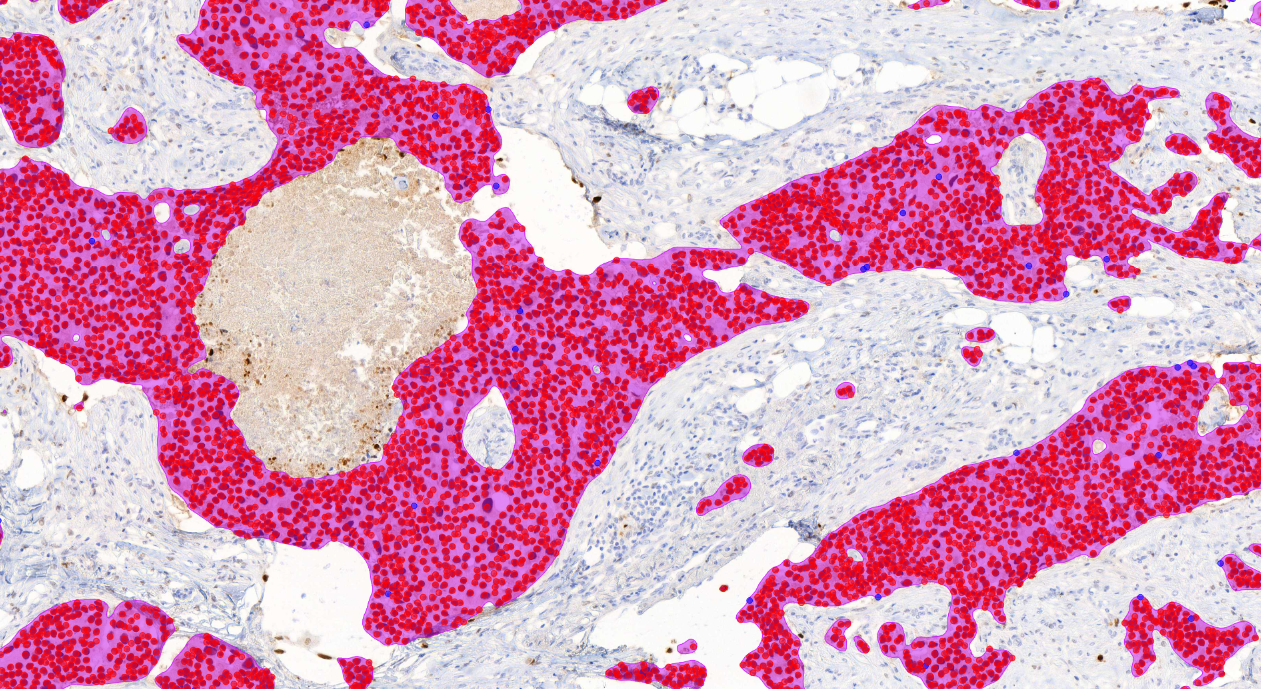
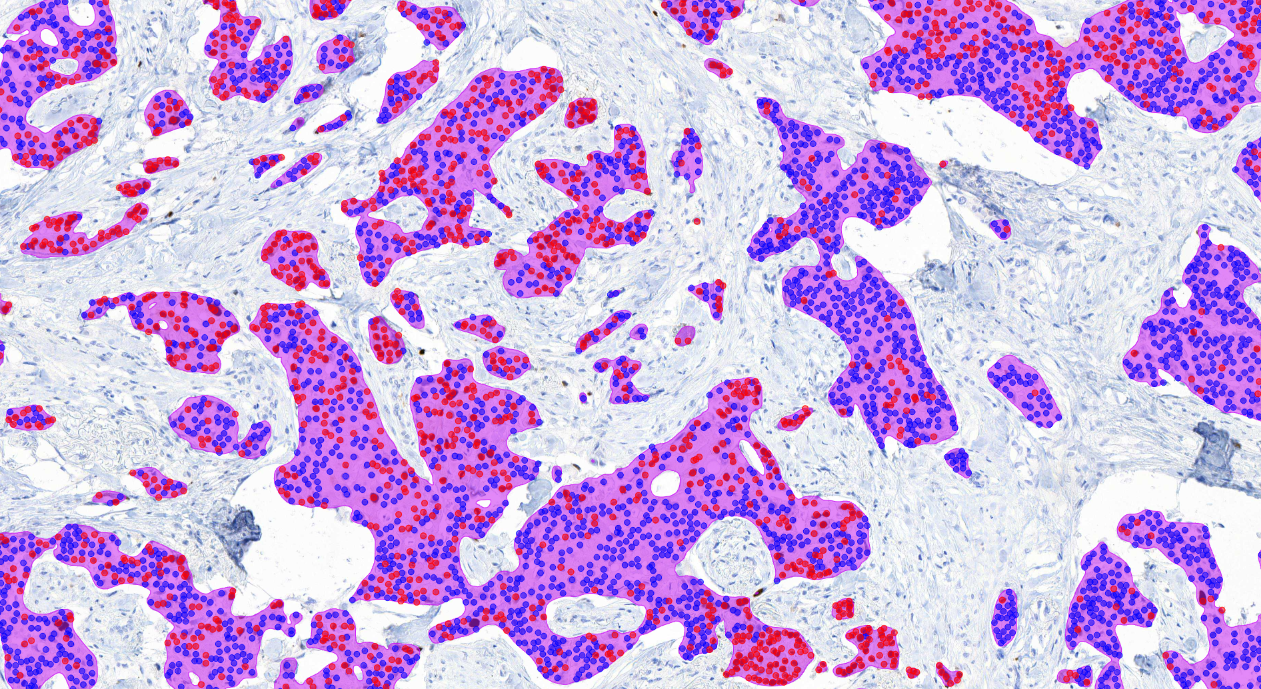
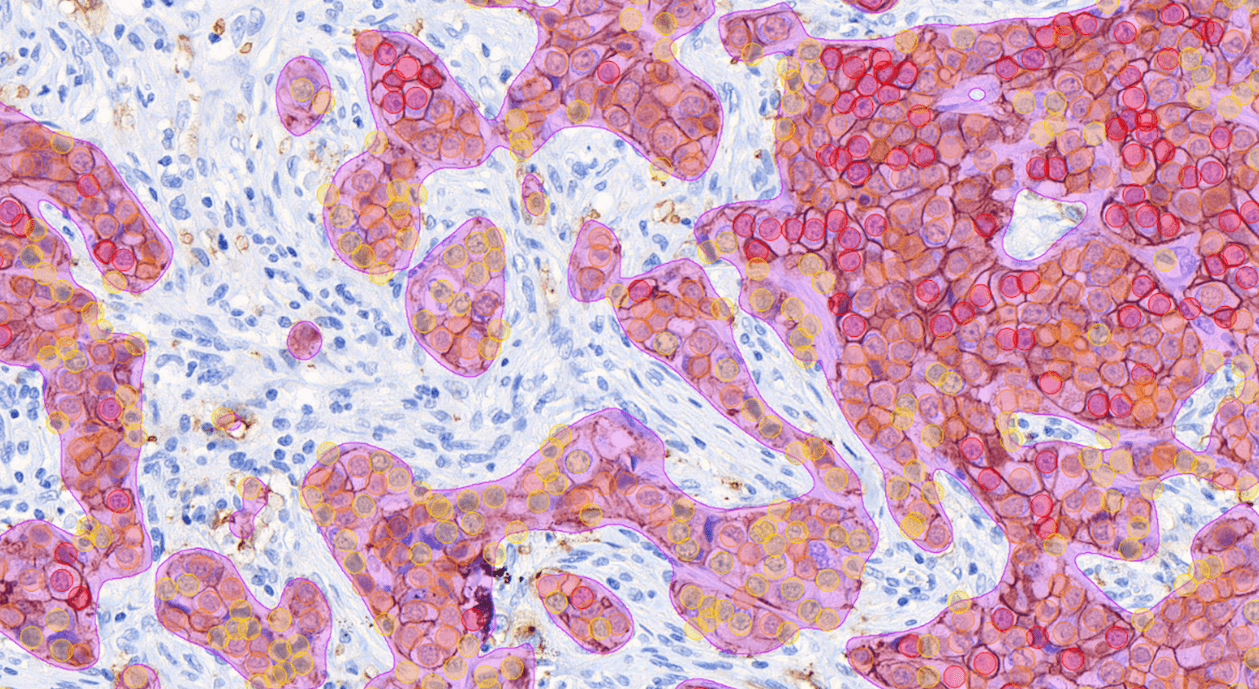
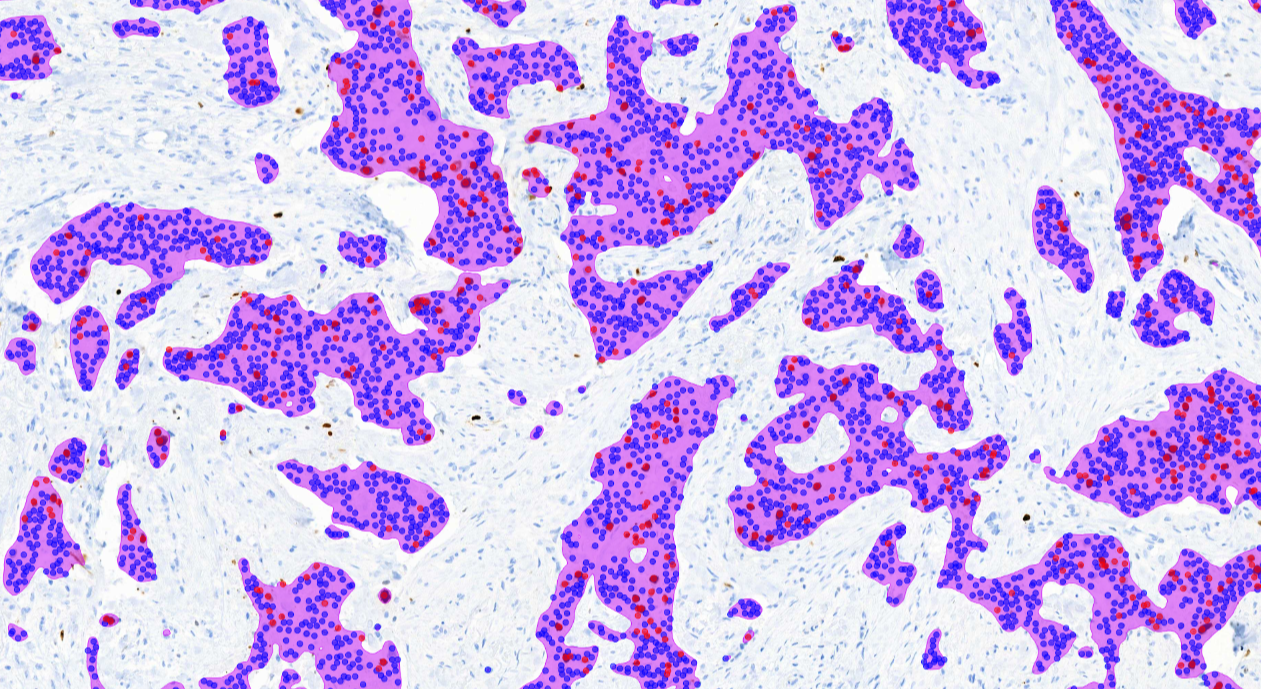
Aiforia® Breast Cancer Suite consists of AI models with an optimized interactive user interface that supports pathologists in the histological grading of breast cancer and in the assessment of breast cancer IHC markers.
Only certain Aiforia® Clinical AI models and the Aiforia® Clinical Suite Viewer are CE-IVD marked for diagnostic use in EU and EEA countries; see details under each AI model.
Contact us