Worldwide, the most common cause of cancer mortality in 2020 was lung cancer, with an estimated 1.80 million deaths occurring1. Fortunately, immunotherapies are increasing in prominence and efficacy2. A popular target for these novel therapeutics is PD-L1, a vital biomarker indicating cancer progression3. The calculation of PD-L1 presence in tumors is routinely used in clinical pathology as a predictive diagnostic test and to identify which patients would benefit most from immunotherapy, such as in cases of non-small cell lung cancer (NSCLC), the most prevalent form of lung cancer4.
The calculation of PD-L1 with traditional methods is slow, cumbersome, and prone to variability5. As cancer rates continue to rise, the demand for new, more precise technologies to assist in diagnostics is expanding. The CE-IVD marked Aiforia® Lung Cancer PD-L1 is intended for use by pathologists in supporting them to detect and calculate the levels of the biomarker in non-small cell lung cancer cases. The clinical grade deep learning artificial intelligence model is able to automatically detect the tumor areas in a patient sample and then calculate, or score, PD-L1 negative and positive cells.
“The accurate evaluation of PD-L1 levels in lung cancer is crucial because many patients can become resistant to standard therapies or certain expensive immunotherapies could be ineffective for them. Therefore methods to assist in the precise calculation of this biomarker by pathologists are incredibly important and will lead to more personalized treatment programs for cancer patients,” explains Dr. Juuso Juhila, Aiforia’s Director of Clinical Products.
Aiforia’s latest clinical AI model highlights cancerous areas in patient samples for the pathologist to review. Using Aiforia’s browser-based software, the pathologist is then able to automatically calculate PD-L1, even in cases of weak expression of the biomarker or small foci of cancer, areas which, if evaluated with traditional methods, would often need further studies or second opinions to be made. With Aiforia’s software, the user can quickly locate, zoom in, and review the critical areas to confirm the AI model’s evaluation.
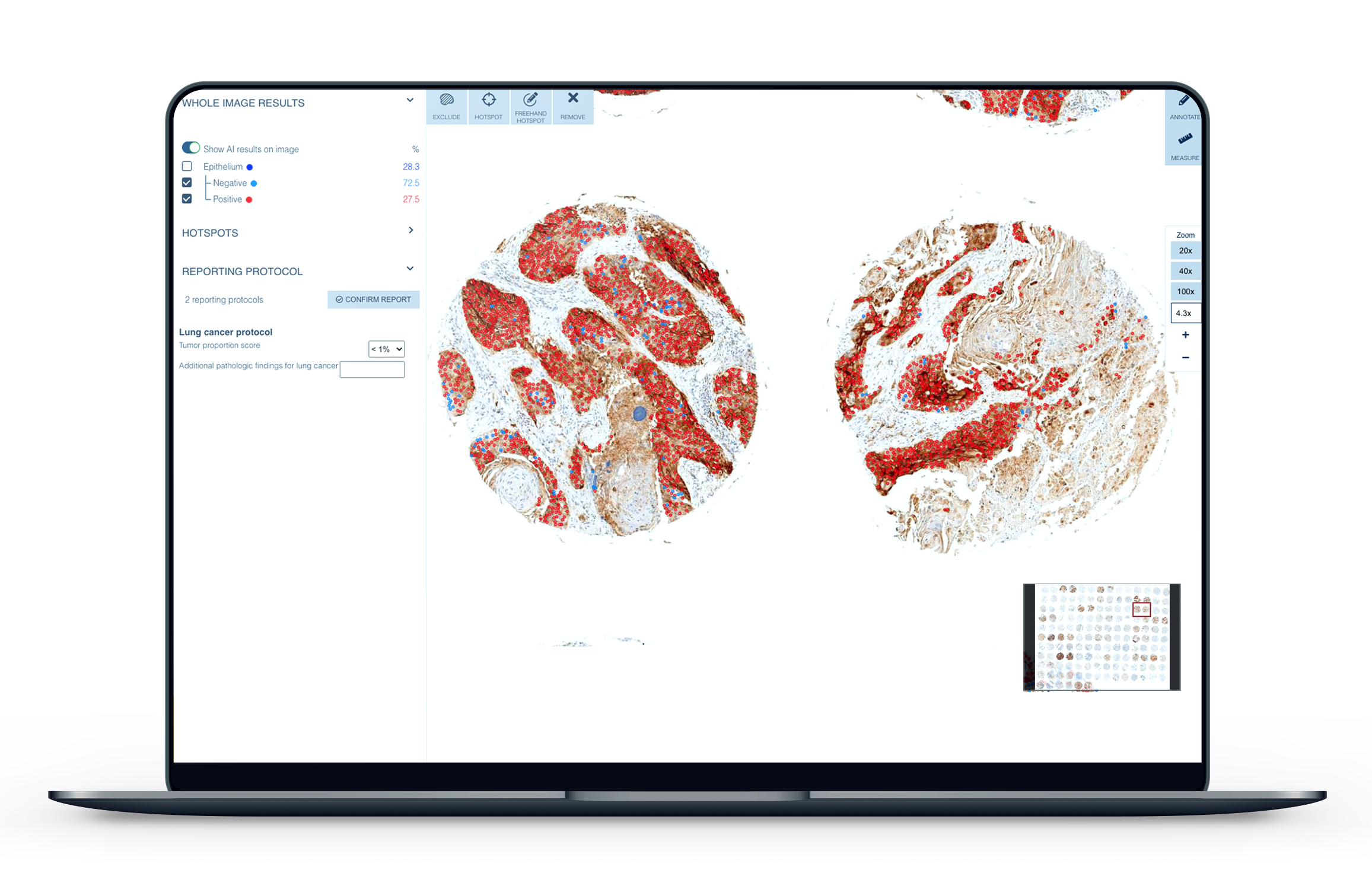
“PD-L1 scoring is a burdensome task which has a direct clinical impact. Therefore the ability to quickly provide an accurate and reproducible score for each patient will be a benefit to pathologists, oncologists, and most importantly, patients,” explains Dr. Anna Laury, Consulting Pathologist at Aiforia.
Aiforia’s team of pathologists from a wide range of subspecialties are working closely with Aiforia’s software team in developing the Aiforia Clinical Suites, a portfolio of tools for AI-supported diagnostics, intelligent visualization of patient samples, as well as automated screening and reporting tools to assist pathologists in the diagnosis of some of the world’s most prevalent cancers such as lung, breast, and prostate cancer. The Clinical Suites are made by pathologists, for pathologists, and aim to enable personalized and democratized care for patients.
References
1. World Health Organization (2021). Cancer. https://www.who.int/news-room/fact-sheets/detail/cancer
2. Garon, E., B. et al. (2015). Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. The New England Journal of Medicine, 372(21), 2018-2028. https://doi.org/10.1056/nejmoa1501824
3. Iwai, Y. et al. (2017). Cancer immunotherapies targeting the PD-1 signaling pathway. Journal of Biomedical Science, 24(26). https://doi.org/10.1186/s12929-017-0329-9
4. Teixidó, C. et al. (2018). PD-L1 expression testing in non-small cell lung cancer. Therapeutic advances in medical oncology, 10, 1758835918763493. https://doi.org/10.1177/1758835918763493
5. Takada, K. et al. (2018). The Significance of the PD-L1 Expression in Non-Small-Cell Lung Cancer: Trenchant Double Swords as Predictive and Prognostic Markers. Clinical lung cancer, 19(2), 120–129. https://doi.org/10.1016/j.cllc.2017.10.014
