Expedited by the COVID-19 pandemic and the disruption it caused to healthcare systems, cancer rates are rising worldwide. Yet, diagnostic methods are finding it difficult to keep up.
Early and precise cancer diagnostics are crucial to increasing survival rates, but current techniques and treatments have significant limitations. Cancer misdiagnosis rates have been estimated at 10-20% of cases, sometimes even higher. Many of them are triggered by rare forms of the disease with little research on their diagnostics or treatment.
However, all types of cancer patients are challenging for laboratory testing. Many tests are affected by the patient’s disease or medication. Normal cellular mechanisms involved in inflammation, for example, are hijacked by tumor cells, so markers of usually tracked processes become useless in cancer – particularly as the disease advances. Alternate methodologies are also often required as different cancers bring different challenges.

Cancer diagnostics commonly try to predict disease through tumor markers found on the surface of cells that help differentiate cell types, allowing for specification of the disease and measurement of treatment efficacy. The problem is that some of these biomarkers are more complex than others, with research only just developing the tools to detect them in a robust and reproducible fashion.
For patients with a suspicion of prostate cancer, tumor-associated circulating endothelial cells (tCEC) are one such high-complexity marker. Hidden in the patient’s blood, tCECs have long required tools beyond what was available to make use of them as an unbiased clinical diagnostic marker. Today, a combination of new technologies from X-ZELL and Aiforia are rectifying the situation.
What are tCECs?
Tumor-associated circulating endothelial cells (tCECs) come from the inner lining of a tumor’s blood vessels and play an integral part in vessel formation. There is a well-documented connection between angiogenesis and tumor growth and elevated amounts of tCEC in circulation correlating with disease progression. With tCECs shedding directly from the tumor’s vessels, they could therefore help clinicians detect different types of cancer in their early stages and distinguish between aggressive and non-aggressive forms of the disease – all by analyzing a simple blood sample.
Identifying and measuring tCECs
tCECs are exceedingly rare and were long considered undetectable in routine clinical practice because they carry many common blood cell markers and few specific tumor surface markers – making them almost indistinguishable from healthy blood cells.
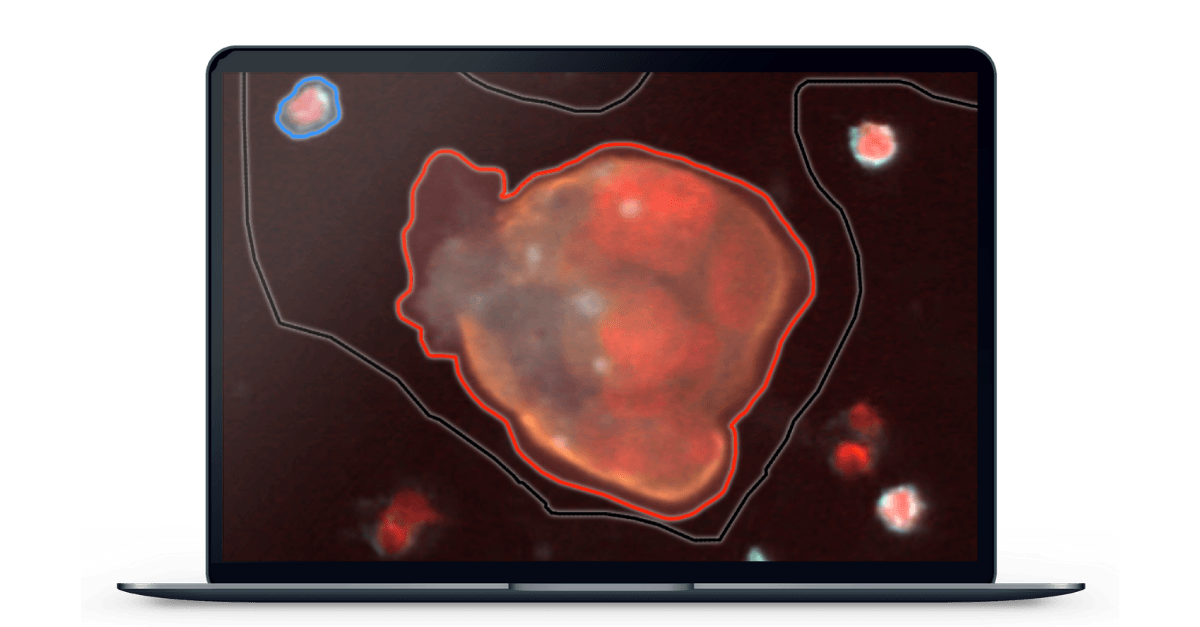
Singapore-based X-ZELL was able to prove otherwise with their patented hMX™ Cell Separation and Cryoimmunostaining™ technologies, which allow them to separate cancerous cells from healthy ones and mark the remaining cells with fluorescently labelled antibodies on a microscope slide to determine whether or not tCEC are present.
Immunofluorescence (IF) is a common research technique. By performing the process at specific sub-zero temperatures, X-ZELL has taken it in a new direction to optimize it for the detection of tCEC. Detecting tCEC is also dependent on increasing the number of fluorophores from a usual 2-3 per slide to 9, allowing for visualization of more complex cells, cell structures, and cell-cell interactions. With X-ZELL’s modular platform technology, it is now possible to detect a single cancer cell among billions of blood cells while keeping both cell morphology and DNA / RNA intact for downstream analysis.
Elevating early detection with AI
While new methods for the detection and visualization of tCECs are a significant step towards better cancer diagnostics, there are still limitations to their clinical adaptability. With multiple antibodies per slide and extremely low numbers of tCECs per sample, it can be laborious and time-consuming for the healthcare professional to analyze the data required to make a diagnosis. Here, Artificial Intelligence (AI) and automated image analysis can provide fast and accurate solutions to make the new technology more broadly accessible.
Aiforia’s AI software is able to analyze a digitized slide at the sub-pixel level and highlight atypical cells to a pathologist for final assessment, thereby speeding up review time and reducing the risk of visual bias. Automating this resource-intensive step will make X-ZELL’s breakthrough technology available to specialists worldwide and enable precision diagnostics in both developed and developing countries.
While prostate cancer has been X-ZELL’s first use case, its tCEC technology may also be applicable to other common cancers. According to the start-up, which has locations in Southeast Asia, Europe and the US, additional tCEC-based blood tests for lung, liver and colon cancer, as well as leukaemia, are already in development.
The goal is a general cancer test capable of identifying tCEC or other circulating atypical cells in a small blood sample. Cross-referencing the visuals with a database of atypical cells could pave the way for non-invasive screening at a population level.
With cancer cases continuing to rise globally, solutions like these could not only help us catch up on the time lost due to COVID-19 but also make cancer diagnostics more widely accessible, safer, and reliable.
References
Bhakdi, S. C. et al. (2019). Accuracy of Tumour-Associated Circulating Endothelial Cells as a Screening Biomarker for Clinically Significant Prostate Cancer. Cancers, 11(8), 1064. https://doi.org/10.3390/cancers11081064
Newman-Toker, D. et al. (2021). Rate of diagnostic errors and serious misdiagnosis-related harms for major vascular events, infections, and cancers: toward a national incidence estimate using the “Big Three”. Diagnosis, 8(1), 67-84. https://doi.org/10.1515/dx-2019-0104